This page contains answers to frequently asked questions about opening, owning, and operating a dental hygiene practice. Topics covered on this page include:
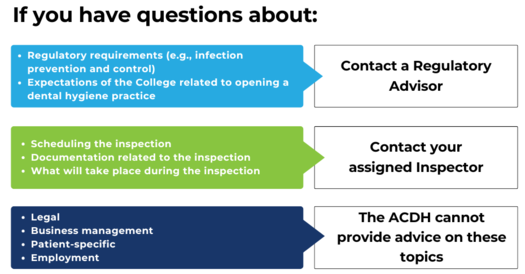
If you have questions about the regulatory requirements or College expectations related to opening a dental hygiene practice, please contact a Regulatory Advisor.
Regulatory Advisors can answer questions about regulation, including scope of practice, Bylaws, Regulations, Standards of Practice and Guidelines, and ethical practice. For example, a Regulatory Advisor can provide you with guidance on Infection Prevention and Control Guidelines in a practice setting.
If you are planning to open a practice, notify the College by filling out the Notification Form for Practice Owners. Then, any questions about scheduling the inspection or what will take place during the inspection can be directed to your assigned Inspector.
Please be advised that the ACDH cannot provide legal, employment, business management, or patient-specific advice.
